

However, the color is made difficult by the strong green also present. This gives a violet-blue color in the presence of excess potassium dichromate(VI) solution. There are several such indicators - such as diphenylamine sulfonate. These change color in the presence of an oxidising agent. formula for Copper (II) Oxide Copper has two possible oxidation numbers. With potassium dichromate(VI) solution you have to use a separate indicator, known as a redox indicator. The oxygen appears to have lost 2 electrons, so its oxidation number is +2. Unfortunately potassium dichromate(VI) solution turns green as you run it into the reaction, and there is no way you could possibly detect the color change when you have one drop of excess orange solution in a strongly colored green solution. As soon as you add as much as one drop too much, the solution becomes pink - and you know you have reached the end point. As you run the potassium manganate(VII) solution into the reaction, the solution becomes colorless. Potassium manganate(VII) titrations are self-indicating. The main disadvantage lies in the color change.

That means that you don't get unwanted side reactions with the potassium dichromate(VI) soution. In the smelting of iron from iron oxide according to the equation FezO3 (s)+3CO (g) a.
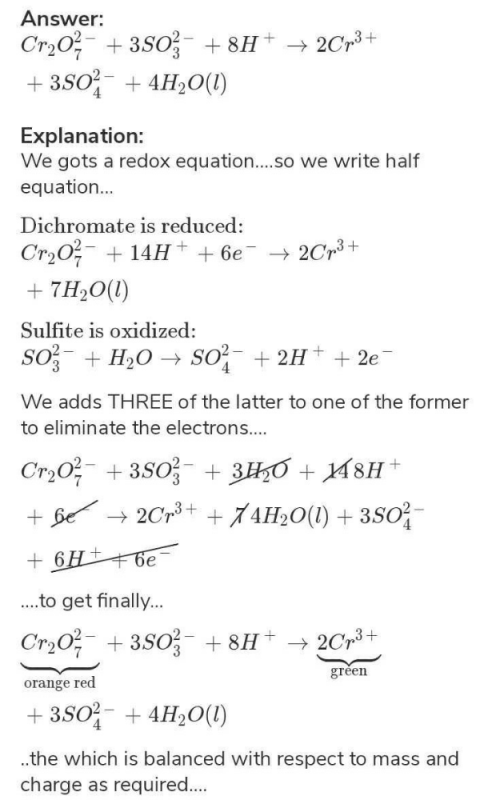
+6 ogeneous mixture of two or more substances is called a. Potassium manganate(VII) oxidises chloride ions to chlorine potassium dichromate(VI) isn't quite a strong enough oxidising agent to do this. What is the oxidation number of chromium in the dichromate ion Croo) a. Potassium dichromate(VI) can be used in the presence of chloride ions (as long as the chloride ions aren't present in very high concentration).That isn't true of potassium manganate(VII). That means that it can be made up to give a stable solution of accurately known concentration. Potassium dichromate(VI) can be used as a primary standard.There are advantages and disadvantages in using potassium dichromate(VI). You will see that the chromium(III) sulfate and potassium sulfate are produced in exactly the right proportions to make the double salt.


 0 kommentar(er)
0 kommentar(er)
